Viral Ghosts: COVID-19 leaves a proinflammatory trace
Written for MicroBites
Although COVID-19 has dominated the conversation around our understanding of coronaviruses, most virus species within this family do not cause severe illness. The majority of human coronaviruses are associated with the common cold, producing symptoms so mild, we rarely see the doctor about it. But every so often, a coronavirus appears that surprises us. So what is different about a coronavirus like SARS-CoV-2 that makes it cause such severe illness?
When a virus is being cleared by our immune systems, enzymes in our cells cut the viral proteins and genetic material into fragments that can then get digested and degraded. However, that may not be the end of those proteins. Once cleaved, the small protein fragments can still interact with host cells. Researchers suspected these protein fragments may mimic proteins that our bodies make in our fight against viral illness.
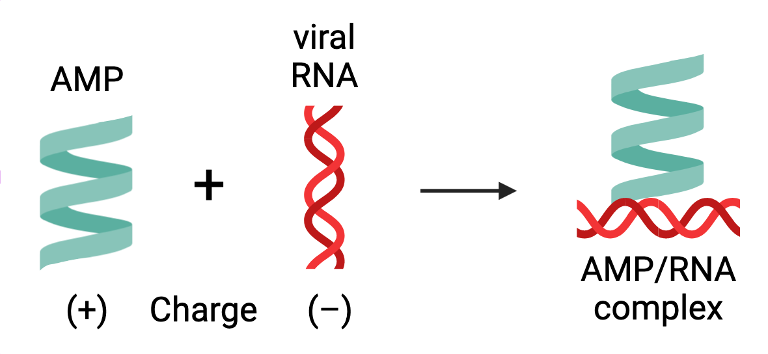
These proteins are called antimicrobial peptides (AMPs). They are short chains of amino acids that can inhibit pathogen growth and infection through a variety of mechanisms. Humans, in particular, have AMPs that fight pathogens by activating the immune system. These AMPs carry a positive charge so that they can attach themselves to viral and bacterial DNA and RNA, which are negatively charged. This AMP-DNA/RNA complex is helpful in instigating our immune response; however, when uncontrolled, it can contribute to autoimmune disorders.
To see if the SARS-CoV-2 proteins can be broken down into peptides that mimic AMPs, the researchers used artificial intelligence trained on known AMP sequences to analyze the virus's genome for AMP-like fragments. Specifically, they searched for sequences that were short in length and a high proportion of positively charged amino acids. The resulting sequences were named "xenoAMPs," xeno- meaning "alien" or "foreign" in reference to their viral origins.
This same sequence analysis on other pathogenic coronaviruses, including SARS-CoV (the causative agent of the SARS outbreak in 2003) and human coronavirus-OC43 (a causative agent of the common cold), yielded a pattern where the more severe the illness associated with a coronavirus, the more xenoAMP sequences were found with machine learning.
To confirm that these peptides actually exist in patients infected with SARS-CoV-2, they tested 29 patients with severe COVID-19, and found 28 of them had viral peptide fragments like the ones predicted with machine learning.
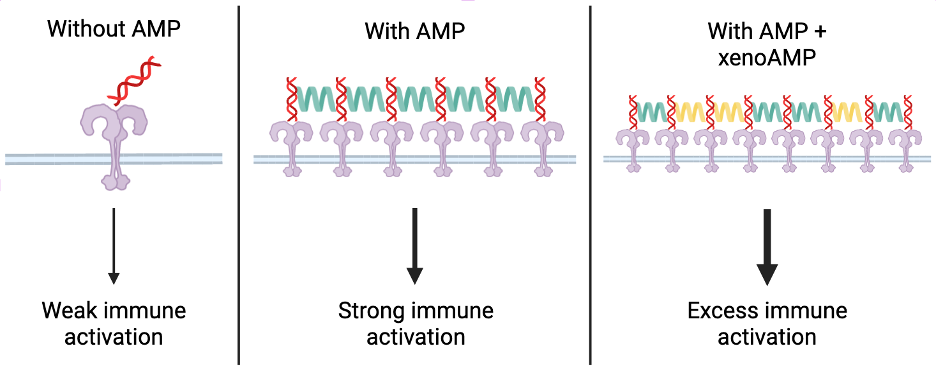
From there, the researchers set out to characterize the xenoAMPs from SARS-CoV-2 as best they could. Artificial xenoAMPs were synthesized from scratch, and compared to the activity of human AMPs. In our cells, AMPs activate the immune system by binding to viral RNA. This RNA-AMP complex helps to amplify the signal that activates our immune system through a receptor called toll-like receptor 3 (TLR-3). When the researchers combined the synthesized xenoAMPs with synthetic viral RNA, they readily formed complexes with each other in the same manner that human AMPs do with viral genetic material in our bodies. In fact, they are so similar that when these xenoAMPs are combined with actual human AMPs, they all come together to form one big complex, suggesting that xenoAMPs worsen infection by over-activating our immune response via this particular TLR-3 pathway.
Because SARS-CoV-2 affects a wide variety of cell types, the researchers tested how many different human cell types respond to the xenoAMP-nucleic acid complexes. In all the cell types, ranging from immune cells to skin cells to heart cells, cells treated with xenoAMP-nucleic acid complexes had the highest immune response compared to an untreated condition and the two complex components alone. The same was observed when healthy mice were infected with the xenoAMP-nucleic acid complexes. In fact, this tiny complex is so effective at inducing an immune response that it almost entirely recapitulates the immune response that cells would have in response to the entire SARS-CoV-2 virus.
XenoAMPs causing so much excess inflammation in such diverse tissue types has the potential to explain the wide variety of symptoms observed in patients infected with SARS-CoV-2. The formation of the xenoAMP-nucleic acid complex prevents the viral proteins from being completely broken down, prolonging the inflammatory effects. The cooperation between xenoAMPs and our actual human AMPs also explains why patients with pre-existing inflammatory conditions experience more severe COVID-19 symptoms since the xenoAMPs work to amplify even further their already hyperactive immune response.
There is still much to learn about this new class of viral molecule, but it is a start in understanding how systemic the effects of SARS-CoV-2 infection really are. This discovery connects COVID-19 symptoms that previously seemed unrelated, and provides insight on why SARS-CoV-2 can cause such severe disease while its cousins in the coronavirus family barely tickle the throat. The next questions have been posed—such as whether xenoAMPs not bound by nucleic acids can do harm and what xenoAMPs are present in patients with varying severity of illness—and the researchers remain in pursuit of the answers.